incomplete octet of electrons|The Incomplete Octet : Manila Ene 30, 2023 — The problem with this structure is that boron has an incomplete octet; it only has six electrons around it. Hydrogen atoms can naturally only have only 2 electrons in their . Elevate your casino experience with our premier charter bus services tailored for the ultimate gaming adventure. Northwest Navigator offers seamless transportation solutions, ensuring you arrive at the hottest casinos in the Portland, Oregon, and Washington areas with ease and comfort.
PH0 · Violations of the Octet Rule
PH1 · The Incomplete Octet
PH2 · The Incomplete Octet
PH3 · Octet Rule Definition, Examples, and Exceptions
PH4 · Incomplete Octet
PH5 · Exceptions to the Octet Rule
PH6 · Exception to the Octet Rule: Examples
PH7 · 9.9: Exceptions to the Octet Rule
PH8 · 8.5 Exceptions to the Octet Rule
Have you ever been in a situation where you accidentally over recharged you simcard with airtime? Yeah I know how painful it is especially when that's the la.
incomplete octet of electrons*******Hun 24, 2021 — The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion.The Incomplete OctetThe problem with this structure is that boron has an incomplete octet; it only has six .
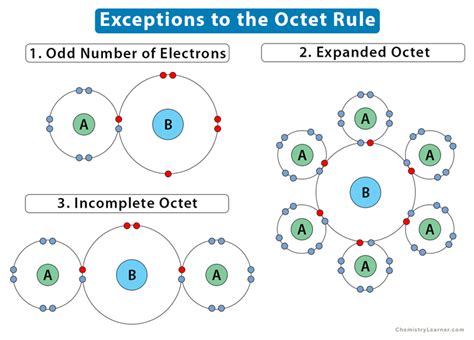
The "octet rule" says that in many compounds the most stable (correct) .incomplete octet of electronsThe "octet rule" says that in many compounds the most stable (correct) .Ene 30, 2023 — The problem with this structure is that boron has an incomplete octet; it only has six electrons around it. Hydrogen atoms can naturally only have only 2 electrons in their .While molecules exist that contain atoms with fewer than eight valence electrons, these compounds are often reactive and can react to form species with eight valence electrons. For .Incomplete Octet. There are certain atoms of certain elements that can exist in stable compounds forming bonds with less than eight valence electrons. When this occurs, the atom of the .
The "octet rule" says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). This is a consequence of the fact that many compounds involve the s and p block .Ago 3, 2019 — Too Many Electrons: Expanded Octets. Todd Helmenstine. Elements in periods greater than period 3 on the periodic table have a d orbital available with the same energy quantum number. Atoms in these periods may .
The Incomplete Octet. While most elements below atomic number 20 follow the octet rule, several exceptions exist, including compounds of boron and aluminum. Learning Objective. .Exceptions to the Octet Rule. Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures. These molecules fall into three categories: Odd-electron .Hun 18, 2023 — The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. This achieves a stable electron configuration similar to that of noble gases.Some common examples of incomplete octet molecules are boron trifluoride (BF 3), beryllium chloride (BeCl 2), and beryllium hydride (BeH 2). Boron (B) has 3 valence electrons, and .Set 21, 2022 — Exceptions to the octet rule fall into one of three categories: (1) an incomplete octet, (2) odd-electron molecules, and (3) an expanded octet. Incomplete Octet. In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be expected to form .An incomplete octet of electrons refers to a situation where an element has fewer than eight electrons in its valence shell. Let’s analyze each option: A. \(BF_3\) (Boron trifluoride): Boron has only six electrons in its valence shell, so it has an incomplete octet.Incomplete Octet. Lighter s- and p-block elements can form compounds with less than 8 valence electrons and hence, have incomplete octets. Hydrogen, lithium, beryllium, and boron have fewer electrons to complete the octet. Helium has 2 electrons in its valence shell but is an inert gas. It does not form a bond with other atoms.
Other articles where incomplete octet is discussed: chemical bonding: Incomplete-octet compounds: Less common than hypervalent compounds, but by no means rare, are species in which an atom does not achieve an octet of electrons. Such compounds are called incomplete-octet compounds. An example is the compound boron trifluoride, BF3, which is used as an .

Hun 18, 2020 — Exceptions to the octet rule fall into one of three categories: (1) an incomplete octet, (2) odd-electron molecules, and (3) an expanded octet. Incomplete Octet. In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be expected to form .
The Octet Rule. The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.This allows each halogen atom to have a noble gas electron configuration, which corresponds to eight valence electrons.
Okt 10, 2023 — For boron trifluoride (BF 3; 24 valence electrons), the analogous structure with an incomplete octet arises ((Lewis structure labeled (i), below). In this case, in principle one of the lone pairs of a terminal fluorine atom could be moved to form a double bond to complete an octet for boron (Lewis structure labeled (ii), below).Although the octet rule is not universal, it is useful in understanding the structures of most organic compounds. The octet rule mainly applies to the second and third-period elements of the modern periodic table. There are mainly three limitations of the octet rule: The incomplete octet of .The bonding in carbon dioxide (CO 2): all atoms are surrounded by 8 electrons, fulfilling the octet rule.. The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.The rule is especially applicable to carbon, .
When it comes to the octet rule, that is true. Exceptions to the octet rule fall into one of three categories: (1) an incomplete octet , (2) odd-electron molecules , and (3) an expanded octet. Incomplete Octet . In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight.Ago 23, 2022 — The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets. Species with incomplete octets are pretty rare and generally are .The octet rule reflects the observation that the most stable ions of many elements have eight electrons in their valence shell for gaining the best possible stability.. Chlorine, for example, typically forms an anion with a charge of \(-1\), .Nob 21, 2023 — The octet rule states that atoms, when forming molecular bonds or atomic ions, can obtain better energy stability by achieving a filled valence shell with eight electrons. It is the formation of .
Here group 3A is OK with having 6 octet electrons, 10 octet electrons for six on 5A, 12 octet electrons from element in Group 6A and then we have 14 and then 16. If we take a look here, the elements that typically have an incomplete OP tech are hydrogen, helium, beryllium and then in Group 3 as boron, aluminum, gallium and Indium.
Exception 2: Incomplete Octets. The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets.Dis 10, 2023 — Exception 2: Incomplete Octets. The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets.Exception 2: Incomplete Octets. The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets.While all chlorine atoms reach the octet, aluminum gets only 6 valence electrons — an incomplete octet. Although aluminum chloride is stable, it reacts with molecules like ammonia that have an unshared pair of electrons. The nitrogen in ammonia donates its lone pair to aluminum, forming a special bond called a coordinate covalent or dative .
Furthermore, hard disk drives are usually bigger as their platter size can range from 2.5 to 3.5 inches. Also, the capacity of the mechanical disks is comparatively lower (up to 20 TB) than that of the electrical ones (up to 100 TB). Since SSDs hold much more advantages, we highly recommend getting one if you plan to upgrade your laptop’s .
incomplete octet of electrons|The Incomplete Octet